The Hindu | 9 February 2017
TPP is dead, but its legacy lives on
by Arjun Jayadev
The Trans-Pacific Partnership was dead long before Donald Trump signed his executive order. But its damaging aspects, like stringent IP provisions, have just migrated to other agreements
U.S. President Donald Trump’s first week in office was, to put it mildly, tumultuous. In the midst of the complete upending of normal politics, reflected by developments like the travel ban on seven Muslim-majority countries and the promotion of ‘alternative facts’, it is easy to forget that one of his first acts as President was to veto U.S. participation in the Trans-Pacific Partnership (TPP), a trade deal among 12 Pacific economies that was a decade in the making.
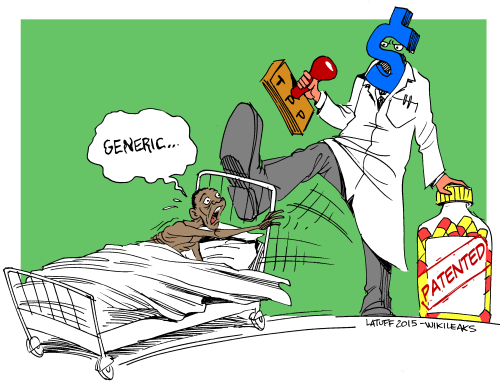
Ostensibly a ‘free trade’ deal, the TPP was opposed extensively by progressives for the last two years because of its far-reaching provisions that increased corporate power over trade at the expense of workers and consumers. The agreement’s damaging ambitions were most evident in the proposed provisions concerning intellectual property.
The TPP provided explicit protections for ‘biologics’ (drugs manufactured in a living organism, rather than through chemical synthesis), the first trade agreement to do so. More damagingly, the agreement mandated the protection of clinical test data submitted for marketing approvals, with pharmaceutical data obtaining five to eight years of protection. This provision, called ‘data exclusivity’ or ‘marketing exclusivity’, prevents a generic company from relying on the clinical test results of the originator in order to prove the efficacy of its drug. It was justified using the argument that clinical trials are the most expensive part of drug development and hence there is a necessity to provide drug developers the ability to limit access to that data so as to incentivise research.
Undermining accessibility
Though, on the surface, the provision looks reasonable, data exclusivity is a deeply uncompetitive policy that serves to undermine generic competition in a troubling way. In fact, it is possibly a stronger restriction than patent protection itself. Patent protection can be challenged if the product is not sufficiently novel, or violates existing national standards for obtaining patent protection, thereby clearing the way for generic competition. (This is, in fact, what happened to Novartis in India over Gleevec, an anti-cancer drug). Simultaneously, generic companies can, and do innovate around patents and produce chemical entities that have the equivalent efficacy of the original drug. But for the generic version to be able to come to the market, it is essential that it be able to use the proof of efficacy and safety that is generated by the clinical trial. The restriction on the use of test data would therefore require a generic company to undertake clinical trials by itself, which is both unfeasible (in terms of expense) and unethical (since it would expose patients to trial protocols, during which some patients would have to receive a placebo when a proven cure is available). As a result, in a country like India, even in a situation where there is no patent barrier, data exclusivity would allow for a period of five to eight years during which there is no plausible way that market access could be allowed for generics, thereby reducing access to cheaper medicines for the population.
The provisions on biologics and data exclusivity in the TPP accompanied others — like those extending patent terms beyond 20 years; weaker patent standards that would allow a greater number of secondary or ever-greening patents on pharmaceuticals; and harsher intellectual property enforcement. Leading public health organisations termed the TPP the worst trade agreement on access to medicines.
The legacy of the TPP leads one to reflect on what it says about U.S. trade policy, particularly as it relates to public health and intellectual property.
First, the developed world’s ambitions for intellectual property will not die with the agreement. Indeed, what seems to be likely is that these damaging provisions will simply migrate to other agreements. One of them is the Regional Comprehensive Economic Partnership (RCEP) agreement which involves 16 countries. The demise of the TPP has been characterised by the media as a boost for China’s trade ambitions in the region, while countries negotiating RCEP appear to be buoyed by the notion that it would now be the largest free trade agreement in existence; they are proposing a rapid conclusion to the talks, perhaps even as early as in the coming months. Courtesy of Japan and South Korea, the RCEP negotiations feature several of the intellectual property provisions of the TPP. This should be of great concern for access to medicines globally, as countries involved in the RCEP negotiations include key generic drug-producing countries, including India.
No change in U.S. approach
Second, U.S. withdrawal from the TPP may change the U.S.’s approach to trade and intellectual property more in form than in substance, by switching from trade agreements that include several countries to bilateral free trade agreements (FTAs). In this process, the U.S. is more than likely to continue its vigorous campaign against perceived “violators” of U.S. intellectual property. The pressure exerted by the Obama administration on the public health safeguards in Indian patent law over the past eight years are likely to continue, if not worsen.
Third, despite the public health impact of the TPP’s provisions having gripped public discussion on the agreement, it is unlikely that these concerns will guide U.S. trade or foreign policy. President Trump’s remarks in early January emphasised his desire to end “global freeloading” stating that “foreign price controls reduce the resources of American drug companies to finance drug and R&D innovation... our trade policy will prioritise that foreign countries pay their fair share for U.S.-manufactured drugs, so our drug companies have greater financial resources to accelerate development of new cures.”
In reality, the TPP had been dead long before President Trump’s executive order was signed. Still, it is evident that the stealthy global subversion of access to medicines through trade agreements and diplomatic pressure by the U.S. will continue. With these efforts spilling across the developed world and into multiple trade agreements, there is now an even greater need for vigilance against the ever-expanding corporate control over medicines. In India, we will need far greater government commitment to the use of the public health safeguards in our patent law to survive this era and ensure the health of the country’s citizens.
Arjun Jayadev is an associate professor of economics at Azim Premji University and the University of Massachusetts Boston, and part of a Shuttleworth Foundation project on access to medicines.